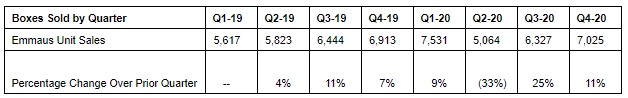
TORRANCE, Calif., Jan. 27, 2021 /PRNewswire/ -- Emmaus Life Sciences, Inc. (OTC: EMMA), a leader in the treatment of sickle cell disease, reports that it sold 25,947 boxes of Endari® in 2020 compared to 24,797 boxes in 2019, an increase of 5%. Emmaus is providing this information in advance of the filing of its Quarterly Reports on Form 10-Q for the first, second and third quarters of 2020 and Annual Report on Form 10-K for the year ended December 31, 2020.
"We are pleased with our increase in sales volume in 2020, particularly in light of industry-wide challenges posed by COVID-19 during the year," said Dr. Yutaka Niihara, M.D., M.P.H., Chairman and Chief Executive Officer. "With the exception of the second quarter of 2020, Emmaus has achieved continuous sequential quarterly growth in boxes of Endari® sold in 2019 and 2020."
Product shipments of boxes of Endari® by Emmaus to its customers are referred to as "Emmaus Unit Sales." In accordance with U.S. GAAP, Emmaus' reported net revenues are determined by adjusting gross sales for shipments in transit, fees, discounts, rebates, other variable consideration, and adjustments to prior period estimates of variable consideration. The wholesale acquisition cost (WAC) per box of Endari® was $1,110 in 2019 and increased to $1,154 effective January 1, 2020. The cost to patients is reduced by any applicable co-pay assistance programs and eligibility for certain of Emmaus' patient assistance programs.
The following table summarizes Emmaus Unit Sales for 2019 and 2020:
About Emmaus Life Sciences
Emmaus Life Sciences, Inc. is a commercial-stage biopharmaceutical company engaged in the discovery, development, marketing and sale of innovative treatments and therapies, including those in the rare and orphan disease categories. For more information, please visit www.emmausmedical.com.
About Endari® (prescription grade L-glutamine oral powder)
Indication (U.S.) - Endari® is indicated to reduce the acute complications of sickle cell disease in adult and pediatric patients five years of age and older.
Important Safety Information
The most common adverse reactions (incidence >10 percent) in clinical studies were constipation, nausea, headache, abdominal pain, cough, pain in extremities, back pain, and chest pain.
Adverse reactions leading to treatment discontinuation included one case each of hypersplenism, abdominal pain, dyspepsia, burning sensation, and hot flash.
The safety and efficacy of Endari® in pediatric patients with sickle cell disease younger than five years of age has not been established.
For more information, please see full Prescribing Information of Endari® at: www.ENDARIrx.com/PI.
About Sickle Cell Disease
Sickle cell disease is an inherited blood disorder characterized by the production of an altered form of hemoglobin which polymerizes and becomes fibrous, causing red blood cells to become rigid and change form so that they appear sickle shaped instead of soft and rounded. Patients with sickle cell disease suffer from debilitating episodes of sickle cell crises, which occur when the rigid, adhesive and inflexible red blood cells occlude blood vessels. Sickle cell crises cause excruciating pain as a result of insufficient oxygen being delivered to tissue, referred to as tissue ischemia, and inflammation. These events may lead to organ damage, stroke, pulmonary complications, skin ulceration, infection and a variety of other adverse outcomes. Sickle cell disease is a significant unmet medical need, affecting approximately one hundred thousand patients in the U.S. and millions worldwide, the majority of which are of African descent. An estimated 1-in-365 African American children are born with sickle cell disease.
Forward-looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding sales trends. These forward-looking statements are subject to numerous assumptions, risks and uncertainties which change over time, including uncertainties related to Emmaus' working capital and ability to carry on its existing operations and obtain needed financing and other factors previously disclosed in the company's reports filed with the Securities and Exchange Commission, and actual results may differ materially. Such forward-looking statements speak only as of the date they are made, and Emmaus assumes no duty to update them, except as may be required by law.
SOURCE Emmaus Life Sciences, Inc.

Related Links
